Hydrogen Background
Contents
- 1 'Types' of Hydrogen
- 2 Hydrogen Production Processes
- 3 Green Hydrogen
- 3.1 The Electrolytic Process
- 3.2 Sea Water as a Source of Electrolysis Water
- 3.3 'Produced' Water as a Source for Electrolysis Water
- 3.4 Fracking Water as a Source for Electrolysis Water
- 3.5 Electrolyzer Installations in the US
- 3.6 Falling Costs
- 3.7 Worldwide Plans for Electrolyzer Factories
- 3.8 Green Hydrogen Companies
- 3.9 Green Hydrogen in Residential Applications
- 4 Blue Hydrogen
- 5 White (Natural or Geologic) Hydrogen
- 6 Purple Hydrogen (Bio-Hydrogen, Biomass Hydrogen)
- 7 Water Consumption of 'Green' vs 'Blue' Hydrogen
- 8 Oxygen: a Byproduct of Electrolysis (Green Hydrogen)
- 9 Hydrogen - A Greenhouse Gas too?
- 10 Town Gas
- 11 Natural Gas
- 12 Industrial Hydrogen Uses
- 13 Hydrogen As a Fuel
- 14 Hydrogen Storage
- 15 Hydrogen Pipelines
- 16 Hydrogen Safety
- 17 Hydrogen Markets
- 18 Annual Water Consumptions in NM
- 18.1 2015 NM Water Usage Categories
- 18.2 What Impact Will Cannabis Growers Have on Water Usage?
- 18.3 How Much Water Will Regenerative Agricultural Practices Save?
- 18.4 2015 NM State Engineer Water Usage Numbers
- 18.5 2005 NM State Engineer Water Usage Numbers
- 18.6 Problems with Oil & Gas Field Waters
- 19 Hydrogen Hubs Background
- 20 Hydrogen Industry Conferences
- 21 Special Interest Groups on Hydrogen
- 22 Summary
- 23 Physical Properties
- 24 Abbreviations
'Types' of Hydrogen
Hydrogen itself is a colorless, odorless gas. It is the most abundant element in the universe and forms water when burned or reacted with oxygen.
Hydrogen 'colors' disguises the impact GHG emissions have with hydrogen production from different manufacturing processes. The hydrogen color spectrum can be summarized as:
- Green - hydrogen made from renewable sources, essentially the electrolytical processes, using water and renewable energy such as wind and solar.
- Blue - gray hydrogen with carbon capture and sequestration processing added.
- Gray - hydrogen made from the methane in natural gas and other oil and gas products, mostly using Steam Methane Reforming.
- Brown - hydrogen made from coal mostly via Coal Gasification.
- Pink - hydrogen via electrolysis of water using energy from nuclear power.
- Turquoise - hydrogen made using an unproven process called methane pyrolysis to produce hydrogen and solid carbon.
- Yellow - hydrogen made through electrolysis using only solar power.
- White - hydrogen is a naturally-occurring geological hydrogen found in underground deposits.
- Gold - another name for naturally-occurring hydrogen.
- Orange - hydrogen made by artificially injecting water into suitable geological strata where it reacts with minerals, mostly iron minerals to make hydrogen and metal oxides, see White hydrogen.
- Purple - hydrogen made from biomass fermentation (our suggested color)
'Clean' hydrogen is an ambiguous phrase meant to refer to hydrogen production that has a net-zero or better GHG emissions 'footprint'. Sometimes it is used to refer to hydrogen made with low but not insignificant CO2 emissions, compared to fossil-fuel hydrogen. The US 2022 Inflation Reduction Act, 45V Credit, as reported by the DoE Clean Hydrogen Production Standard (CHPS), defines "qualified clean hydrogen" as hydrogen produced “through a process that results in a lifecycle greenhouse gas emissions rate of not greater than 4 kilograms of CO2e per kilogram of hydrogen.” On site production that achieves less than 2 kilograms of CO2e per kilogram of hydrogen are expected to achieve the lifecycle target. Down-stream emissions from the use of such hydrogen are not included.
Natural hydrogen is found in geological strata. 'White' and 'gold' hydrogen fall into this category.
Renewable hydrogen is made from renewable energy and water. 'Yellow, 'green' and 'purple' hydrogen fall into this category. 'Orange' hydrogen may also be included if the injection process is run on renewable energy.
Fossil-fuel hydrogen or fossil-hydrogen is made mostly from methane in natural gas and other oil and gas products. 'Blue', 'gray', 'brown' and 'turquoise' hydrogen fall into this category.
Note, until all manufacturing, storage and transportation industries and so on have been decarbonized, all hydrogen production will have some life-cycle carbon footprint.
Hydrogen Production Processes
Steam Methane Reforming
Most of the 10 Mtpa of the hydrogen produced in the US is generated from methane gas in the Steam Methane Reforming process. The process uses water as steam to generate hydrogen from the reactions:
CH4 + H2O => CO + 3 H2O
CO + H2O => CO2 + H2
CH4 + 2 H2O => CO2 + 4 H2O
Energy has to be provided to maintain high temperatures.
Electrolysis of Water
A DC electrical power source is connected to two electrodes, or two plates (typically made from an inert metal such as platinum or iridium) which are placed in water. Hydrogen appears at the cathode (where electrons enter the water), and oxygen appears at the anode. Assuming ideality, the molar amount of hydrogen generated is twice the molar amount of oxygen, and both are proportional to the total electrical charge conducted by the solution. However, in many cells competing side reactions occur, resulting in different products and less than ideal efficiency. On a mass basis, 8 kg of oxygen are produced for every kg of hydrogen.
Electrolysis of pure water requires excess energy in the form of overpotential to overcome various activation barriers. Without the excess energy, the electrolysis of pure water occurs very slowly or not at all. This is in part due to the limited self-ionization of water. Pure water has an electrical conductivity about one-millionth that of seawater. Many electrolytic cells may also lack the requisite electrocatalysts. The efficiency of electrolysis is increased through the addition of an electrolyte (such as a salt, an acid or a base) and the use of electrocatalysts.
Solar Thermochemical Production
An emerging water-splitting technology called solar thermochemical hydrogen (STCH) production, which can be potentially more energy efficient than producing hydrogen via the commonly used electrolysis method is in development at the National Renewable Energy Laboratory. STCH relies on a two-step chemical process in which metal oxides are exposed to temperatures greater than 1,400 C and then re-oxidized with steam at lower temperatures to produce hydrogen. Like electrolysis, the system produces byproduct oxygen. See:
- System and technoeconomic analysis of solar thermochemical hydrogen production - Renewable Energy - May 2022
Green Hydrogen
The Electrolytic Process
Green hydrogen is manufactured through a process of electrolysis powered by renewable energies such as wind or solar. Electrolysis of water passes a direct electrical current through water containing some salts, in a cell with two electrodes an anode and a cathode. The cell is known as an 'electrolyzer'.
Oxygen shows up at the anode and hydrogen at the cathode and when the two are connected together an electric current flows from anode to cathode in an external circuit. Catalysts on the electrodes are in development to make the process more and more efficient.
Two main types of electrolyzers are used in green hydrogen production: alkaline electrolyzers, which use a liquid alkaline electrolyte, typically potassium hydroxide, and proton exchange membrane (PEM) electrolyzers, which use a solid polymer membrane.
Notably, the process has no carbon emissions.
Sea Water as a Source of Electrolysis Water
"Seawater split to produce green hydrogen" - the Feb 2023 article discusses recent work from researchers at the University of Adelaide. THey claim to have developed a catalytic process with close to 100% conversion that makes hydrogen via electrolysis from untreated sea water. The application to other saline solutions may be next.
"A step closer to sustainable energy from seawater" article from phys.org Aug 2018 talks about a catalyst that reduces the amount of chlorine produced when using seawater in electrolysis. Presumably brine (saltwater) would have the same problem. Properly captured and separated/managed chlorine has the potential to be used in other products (chemicals such as bleach).
From Wikipedia on Chlorine:
"In industry, elemental chlorine is usually produced by the electrolysis of sodium chloride dissolved in water. This method, the chloralkali process industrialized in 1892, now provides most industrial chlorine gas. Along with chlorine, the method yields hydrogen gas and sodium hydroxide, which is the most valuable product."
From the article "China set to drive global chlorine capacity by 2024"
"The global chlorine capacity is poised to see moderate growth over the next five years, potentially increasing from 87.69 million tons per annum (Mtpa) in 2019 to 92.13 Mtpa in 2024, registering total growth of 5%"
The chlor-alkali process is electrolysis of sodium chloride solutions (e.g. brine) to produce hydrogen, chlorine and sodium hydroxide (the alkali bit). Stoichiometrically, for every molecule of chlorine produced there is one molecule of hydrogen produced. So 87.69 Mtpa of 2019 chlorine (atomic weight 35.45) should produce 87.69/35.45 = 2.47 Mtpa of hydrogen. Does the US really need anymore chlorine?
'Produced' Water as a Source for Electrolysis Water
Sources
Produced water is naturally occurring water that rises with oil and gas streams from the rocks below and sometimes referred to as 'formation water'.
By 2050 the projected US demand of 41 Mtpa H2 (four times today's) will consume 41 * 18/2 Mtpa water i.e. 369 Mtpa water whether by electrolysis or Steam Methane Reforming.
The Permian Basin is estimated to generate 32 million barrels of produced water per day in 2025 (SFNM). Much is disposed of by reinjecting into wells. In a year with say 10% downtime, that's 10.5 billion barrels/yr, or 32 million barrels/yr * 42 US gals/barrel is 1.386 billion gals/yr.
The current 10.5 billion barrels produced water/yr x 42 US gals/barrel x 8.33 lb/US gal / 2204.6 lb/tonne is equal to 1,666 Mtpa of water. This Permian Basin produced water is more than 4 and half times the water consumption needed for 2050 US hydrogen production via electrolysis or SMR.
The Permian Basin 2025 estimated produced water at 1.386 billon gals/yr compares with 14 billion gals/yr used in fracking in New Mexico.
Produced water could be used as a source for electrolysis water but it is generally highly contaminated and relies on the continued operation of oil and gas fields to be generated. Before it could be used for electrolysis it will need to be decontaminated to prevent damage to electrolyzer cells. Oil and gas wells produce:
- crude oil (a mixture of liquid hydrocarbons)
- natural gas (a mixture of gaseous hydrocarbons)
- sand and other mineral solids in suspension.
- water (containing salinity and other dissolved solids and hydrocarbon contaminants)
The American Geosciences Institute reports that:
- The composition of produced water depends on the chemistry of the rocks it has been in contact with. In the Bakken (North Dakota) and Marcellus (Pennsylvania and neighboring states) formations, produced waters can be over 10 times more saline than seawater. In California and Wyoming, many produced waters are much less saline than seawater. Produced water can also contain varying amounts of oil residues, sand or mud, naturally occurring radioactive materials, chemicals from frac fluids, bacteria, and dissolved organic compounds. Differences in composition affect how produced waters are treated, used, and/or disposed. The U.S. Geological Survey maintains a database of produced water compositions based on over 165,000 measurements across the United States.
'Produced' Water Treatment Options
A variety of processing steps could be used to clean up produced water as described in the paper:
- Review of oilfield produced water treatment technologies - Chemosphere - July 2022
The paper includes a review of worldwide produced water quantities along with their major constituents, regulations, etc. Hydraulic Fracking species are not mentioned as part of the analysis and only referred to as chemical additives probably due to their proprietary nature.
Processing steps discussed in the Chemosphere piece can involve chemical and physical operations and include:
Primary Treatments
- Hydrocyclones
- Coagulation and flocculation
- Oil-water separators
Secondary Treatments
- Flotation
- Adsorption
- Activated sludge process
- Electrodialysis
- Freeze-thaw/evaporation
- Macro-porous polymer extraction technology (MPPE)
- Membrane treatment technology
- Microfiltration
- Ultrafiltration
- Nanofiltration
- Reverse osmosis
- Advanced oxidation processes
- Ozonation
- Fenton process
- Photocatalytic oxidation process
Processes selected depend entirely on the nature of the water encountered and its contaminants which can vary considerably.
'Produced' Water Radioactivity
Radioactive components in produced water are discussed in the EPA article on Technologically Enhanced Naturally Occurring Radioactive Material (TENORM):
The geologic formations that contain oil and gas deposits also contain naturally-occurring radionuclides, which are referred to as Naturally Occurring Radioactive Materials (NORM):
- Uranium and its decay products.
- Thorium and decay products.
- Radium and decay products.
- Potassium-40.
- Lead-210/Polonium-210.
Radium levels in the soil and rocks vary greatly, as do their concentrations in other waste streams. Radiation levels may vary from background soil levels more than 4 becquerels per gram (Bq/g), or several hundred picocuries per gram (pCi/g). The variation depends on several factors:
- Concentration and identity of the radionuclides.
- Chemistry of the geologic formation.
- Characteristics of the production process.
Data on radiation levels - relative to background levels - are available from a 1999 USGS report:
The main concern seems to revolve around radium levels.
Fracking Water as a Source for Electrolysis Water
Fracking Water is surface water that is mixed with a cocktail of chemicals and sands and injected into an oil and gas well under pressure to crack or fracture the rock allowing more hydrocarbons to be released. Such Hydraulic Fracturing, known as 'fracking' has to be repeated to keep wells producing. Cutting back on fracking 'releases' water resources for electrolysis.
According to the 2016 Ceres Resaearch Paper, around 100 billion gals/yr are used for hydraulic fracking in the US, equal to 378 Mtpa water, or more than enough for current hydrogen generation demands of about 91 Mtpa water
Flowback water is hydraulic fracturing water that returns to the surface with the oil and gas.
Fracking Water Consumption in New Mexico
A USGS FracFocus report quoted in a Dec 2020 article in the Carlsbad Current Argus, says that in 2019 14 billion gals of water were used in oil and gas production in New Mexico. The State Land Office is also reported to no longer sell freshwater to O&G but how that will be replaced wasn't specified. 14 billion gals of water if electrolyzed could produce 5.88 Mtpa of Hydrogen or over half the US supply:
14,000,000,000 gal/'yr * 8.345 lb/gal / (2.2046 lb/kg * 9 kg H20/kg H2 * 1000 kg/tonne) = 5.88 Mtpa H2
Note that at the current 55 kwh/kg H2, renewable energy needs would be 323 GWh per annum. At 90% on stream time that's a 41 MW power generator or 75 MW unit at 50% OST. Compare that to the Xcel Energy's Sagamore Wind Project in Roosevelt County NM, which is rated at 522 MW!
This is about the same power generation requirements per Mtpa H2 that Fortescue Future Industries claimed above in Plans for GW Electrolyzer Factories.
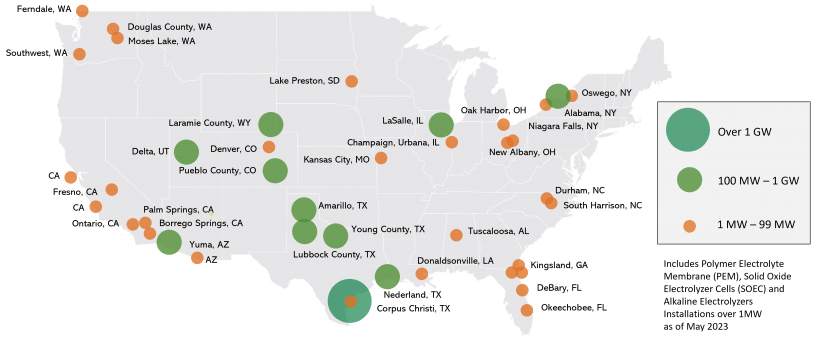
Electrolyzer Installations in the US
As of May 2023 the DoE reported that the planned and existing polymer electrolyte membrane (PEM), solid oxide electrolyzer cell (SOEC), and alkaline electrolyzer installations above 1 MW in the United States were as shown:
The total installed capacity is approximately 67 MW, while planned, firm capacity (with construction under way) is approximately a total of 3.6 GW.
Falling Costs
S&P Global report that falling green hydrogen costs are closing in on fossil-fuel hydrogen costs.
Recharge concurs:
- 'Producing green hydrogen for $1/kg is achievable in some countries by 2030': WoodMac - Recharge - December 2021
Worldwide Plans for Electrolyzer Factories
Electrolyzers are essentially the cells where electrolysis of water is contained producing hydrogen and oxygen. More and more plans have been announced to build factories that in turn make electrolyzer cells.
- 1. S&P Global reports (Nov 9 2021) that the UK company ITM Power is planning to build a second electrolyzer factory to make renewable hydrogen production equipment as it prepares for rapid market expansion. The 1.5 GW/yr facility adds to their existing 1 GW/yr.
- 2. Recharge reported Jan 4, 2022, US company Cummins plans to build a 1GW hydrogen electrolyser factory in China with state-owned oil giant Sinopec.
- 3. Earlier, dated May 24 2021, Recharge reported that Iberdola and Cummins had plans to build a fourth electrolyzer gigafactory in central Spain:
"The €50m ($61m) proton exchange membrane (PEM) electrolyser plant will start up in 2023 in the central region of Castilla-La Mancha, near Madrid, as a 500MW-a-year facility, “and will be scalable to more than 1GW/year”, according to Cummins.
It is the fourth electrolyser gigafactory to be announced in Europe this year, following in the footsteps of the UK’s ITM Power, Norway’s Nel and France’s McPhy. Denmark’s Haldor Topsoe has also unveiled plans for a 500MW plant producing high-efficiency high-temperature solid-oxide electrolysers."
- 4. According to CleanTechnica, US-based Plug Power will partner with Fortescue Future Industries (FFI) to build:
- "World’s Largest Electrolyzer Plant Now Under Construction" (2GW/annum) - CleanTechnica - March 2020
- “The electrolyzer facility located at Gladstone, Queensland, Australia will have an initial capacity of two gigawatts per annum — more than doubling current global production, and enough to produce more than 200,000 tonnes of green hydrogen each year,” FFI writes.
- By 2030, FFI plans to create 15 million tons of green hydrogen a year. That would require more than 200 gigawatts (GW) of new wind and solar generation and a lot more electrolyzers and clean water."
Other electrolyzer manufacturers have also announced giga-scale factories, including Germany’s ThyssenKrupp (5GW), Norway’s Nel (2GW) and France’s McPhy (1GW).
Green Hydrogen Companies
Baofeng Energy
In 2022 ReCharge reported that Baofeng Energy, a Chinese energy company, had fully switched on the world’s largest green hydrogen project, with a 150MW alkaline electrolyser plant, powered by a 200MW solar array.
- Record breaker: World’s largest green hydrogen project, with 150MW electrolyser, brought on line in China - October 2022
Novo Hydrogen
According to their website, US startup NovoHydrogen Holdings LLC received a $20 million equity investment from Modern Energy Group LLC to develop green hydrogen production plants across North America as the US pushes use of the carbon-free fuel. The financial backing “really equips us to keep developing and get that steel in the ground,” Chief Executive Officer Matt McMonagle said in an interview. Novo Hydrogen, a Colorado based company, is dedicated to making renewable hydrogen from electrolysis and renewable energy.
Plug Power
In July 2021, Apex Clean Energy and Plug Power Partner announced the "largest green hydrogen power purchase agreement (PPA) in the United States." 345 MW of power purchased through the PPA will directly supply a new hydrogen production plant with 100% renewable power. The hydrogen plant, which is being co-developed by Apex and Plug Power, will be the first and largest wind-supplied hydrogen project in the United States and the largest onshore wind-powered project across the globe.
- Developers enter largest green hydrogen PPA in US with 345 MW of wind to power facility - Utility Dive - July 2021
Plug Power Inc., 'Plug' is building an end-to-end green hydrogen ecosystem, from production, storage and delivery to energy generation in the US and Europe. Recently Plug Power secured an order for 100 megawatts (MW) of proton exchange membrane (PEM) electrolyzers. This is the largest announced project in the oil and gas sector in Europe [as of July 2023].
- Plug Power Press Release - July 2023
The company is focused on electrolyzer and fuel cell technologies, and vents the oxygen from its electrolyzers to the air.
Green Hydrogen in Residential Applications
According to a report from FuelCellWorks:
On the east coast of Scotland, from next year, about 300 homes in Buckhaven and Methil, in the area of Levenmouth, will be powered by green hydrogen gas. Customers will be offered free hydrogen-ready boilers and cookers in the project, which will initially last five and a half years.
Off the coast at Buckhaven a 200-metre wind turbine will generate electricity to power an electrolyser, which turns water into hydrogen gas and oxygen. The hydrogen will then be stored in pressurised secure tanks, before being pumped into people's homes.
The £28m project, partly funded by the Office of Gas and Electricity Markets (OFGEM), has the capability to be expanded to 1,000 homes from the same turbine. The project hopes to save more than 2,650 tonnes of CO2, which is equivalent to half the homes taking their cars off the road.
Blue Hydrogen
The economics and environmental impacts from 'Blue' hydrogen production, overwhelmingly rely on cost effective and super efficient Carbon_Capture_and_Sequestration (CCS). 'BLue' hydrogen is 'gray hydrogen with CCS added.
350 NM 'Blue' Hydrogen Briefing Nov 2021
A ‘Blue‘ Hydrogen Briefing by Tom Solomon of 350 New Mexico was presented to a meeting of the NMSEA on November 16, 2021. It is now available on YouTube. The 42 min talk itself starts at the 5:30 mark, with Q&A starting at minute 47.
Also the slide deck now includes a new slide on p18 with a second scientific study warning about the climate impacts of producing hydrogen from methane.
Highlights include:
- Blue hydrogen (H2) from methane is a fossil fuel program.
- Blue hydrogen subsidies are fossil fuel subsidies
- The carbon footprint of blue hydrogen is >20% worse than burning fossil natural gas directly. Per two scientific studies.
- Hydrogen is a wasteful energy carrier, 2.3x worse vs electricity.
- Electricity from hydrogen is so much costlier than solar or wind electricity that it is unprofitable.
- Job #1 for the climate is to urgently build wind & solar to replace fossil fuel emissions, not to push hydrogen.
- The draft H2 Hub Act:
- * would allow major CO2 emissions:
- * defines ‘clean hydrogen’ as 2-6 kg of CO2 per kg of H2 produced. Not clean.
- * will increase New Mexico’s carbon footprint.
Union of Concerned Scientists on Hydrogen Policies
The Union of Concerned Scientists (UCS) shared their thoughts in a February 2024 webinar:
Some highlights:
- Hydrogen extends the reach of renewables.
- ‘Blue’ hydrogen needs to be excluded.
- ‘Diverting’ renewable energy to hydrogen that extends fossil-fuel use has to be avoided
- Misuse of the Inflation Reduction Act 45V tax credit might counter-intuitively drive up emissions.
- Pipeline hydrogen mixtures with natural gas are limited to 5-20% hydrogen. [We dispute this since Hong Kong uses town gas with 50% hydrogen. There may be an impact on residential burner requirements at the higher hydrogen concentrations.]
- Hydrogen as a combusted fuel produces NOx. [We dispute this is a problem since there are technical solutions in catalytic converters as used in ICE automobiles today specifically for NOx emissions.]
- Hydrogen has a role in hard to decarbonize industries.
IEEFA on the Economics of 'Blue' Hydrogen Feb 2022
The Institute for Energy Economics & Financial Analysis (IEEFA) has also studied the economics of 'blue' hydrogen:
- Blue Hydrogen: Technology Challenges, Weak Commercial Prospects and Not Green - IEEFA - February 2022
Highlights include:
- Blue hydrogen requires methane; production is energy-intensive
- Blue hydrogen requires carbon capture and storage (CCS)
- Commercial CCS projects have never achieved the industry target rate over time, despite years of efforts
- CCS projects have been very costly
- Cleaner competition has big head start, investments
- Must play catch-up to other technologies, especially batteries
- Blue hydrogen markets likely to shrink due to green competition
IEER on green, gray, blue and pink hydrogen
The Institute for Energy and Environmental Research (IEER) report
confirms most of the points made by others with geologic hydrogen and electrochemical oxygen conspicuous by their absence.
Main recommendations:
- There must be no greenhouse gas emissions in the production of hydrogen from a primary energy source;
- Leaks of hydrogen, the lightest gas, must be kept minimal because hydrogen in the atmosphere has an indirect warming impact – a factor that is so far absent from the proposed definition of “clean hydrogen”, at least in the United States.
- The climate impact of hydrogen leaks and the use of a 20-year warming potential of hydrogen and methane must be incorporated into the “clean hydrogen” standard, to accurately assess the climate impacts of hydrogen and methane leakages when hydrogen is used as an energy source.
- Carbon-free electricity supplying existing loads should not be diverted for hydrogen production.
- No new hydrogen production from fossil fuel feedstocks should be permitted or supported.
- Water equity and justice issues should be fully integrated into hydrogen policies and decision making.
- The use of curtailed renewables for green hydrogen production should be incentivized and safety issues with intermittent production should be addressed with high priority with due attention to safety issues.
- Local and global environmental justice issues should be fully addressed in their local aspects, as well as in the net system balance addressing the benefits of displacing fossil fuels
Main findings:
- Hydrogen leaks have an indirect warming impact; if not minimized they could negate much or all of the climate benefit of using hydrogen.
- Blue hydrogen – made from natural gas with CCS – does not meet the DOE “clean hydrogen” guidance. Blue hydrogen increases net atmospheric methane pollution when replacing fossil fuels Btu-for-Btu unless hydrogen leaks are kept very low and methane leaks are reduced by about two-thirds.
- Diverting carbon-free electricity from existing loads to produce hydrogen results in a net CO2 emissions increase, since fossil fueled electricity will generally be needed to replace a portion of the diverted electricity. In most cases, the resulting net emissions per unit of hydrogen production are higher than emissions from fossil-fuel-based hydrogen production, including grey hydrogen.
- The water intensity of hydrogen production is a major concern and a siting constraint; it raises major water equity and justice issues.
- Green hydrogen used strategically presents major opportunities for decarbonizing the energy system including in making steel and in a variety of uses when made from renewable electricity that would otherwise be curtailed.
- Major environmental justice issues are associated with hydrogen as an energy source, both local and global. There are also environmental benefits when hydrogen reduces fossil fuel use, such as the reduction of fracking and associated pollution.
Rocky Mountain Institute on Hydrogen Jan 2020
- Hydrogen’s Decarbonization Impact for Industry - a Hydrogen Insight Brief from RMI - January 2020
Key Insights
- When considering what a global energy system on a 1.5°C or 2°C pathway will look like by 2050, hydrogen consistently plays a critical role as a low-carbon fuel. In fact, for several of the hydrogen application areas discussed in this Insight Brief, there are no other viable pathways to decarbonization.
- The abatement impact of hydrogen depends strongly on both the specific use case where it is implemented and the way it is produced.
- Hydrogen produced with grid power at the global average carbon intensity – or even with coal gasification – could be used to reduce carbon emissions in steelmaking today.
- Despite lower CO2-intensity than most power grid-based hydrogen sources, there is no long term role for steam methane reform (SMR) in decarbonizing industry sectors unless successfully fitted with carbon capture and storage (CCS).
- In the near-term, electrolysis using Chinese and Indian grid power is less CO2-effective than coal gasification, and EU and US grid power is less efficient than SMR.
- In natural gas-based economies like the United States, the predominantly SMR-based existing hydrogen production plants are quickly on track to become less CO2-efficient than electrolysis.
- Because electrolysis production with grid power will be at parity with SMR within the next 5-year period, EU and US policy should exclusively focus on electrolysis until CCS is a viable and scalable technology.
- Given the long lifetime of hydrogen generation assets, even in coal-heavy economies (such as China and India), any build-out of coal gasification has to be motivated with the belief in CCS retrofit in the 2030 – 2040 timeline.
- Near- and medium-term outlooks for power grid CO2 intensity should be leveraged and implemented as a leading indicator for hydrogen policy.
- The alignment of high-potential for CO2 reduction and the large-scale of off-takers in sectors like steel and shipping, where demand is naturally aggregated in ports, provides a pathway for policy makers to achieve demand at scale. This can significantly accelerate the cost reduction of electrolysis technologies.
White (Natural or Geologic) Hydrogen
Advantages of White Hydrogen
Geologic hydrogen is naturally occurring hydrogen that:
- Accumulates naturally under the ground, generated by geological processes.
- Can be produced using proven engineering practices with minimal environmental impacts and has a small footprint compared to other exploration activities.
- As a replacement for carbon-based fuels, naturally occurring hydrogen offers significant cost and emissions advantages relative to other sources of hydrogen production.
- Geologic Hydrogen does not require fracking or hydraulic stimulation to be produced.
Companies Searching for Geologic Hydrogen
Koloma
Denver-based energy startup Koloma has raised $245.7 million, according to a February 9th 2024 Form D filed with the U.S. Securities and Exchange Commission, a big haul by climate tech standards. The company is developing technology to find and tap underground reservoirs of naturally occurring hydrogen gas. The idea is that geologic hydrogen — also known as “white” or “gold” hydrogen in the industry’s rainbow-inspired jargon — could be mined at lower cost and with a lower carbon footprint than hydrogen produced from fossil fuels or even from renewable electricity.
- Clean hydrogen startup Koloma, using Ohio State tech, uncloaks from stealth mode - Columbus Inno - July 20, 2023
Kaloma reports the following comparisons
| Comparison | Example |
|---|---|
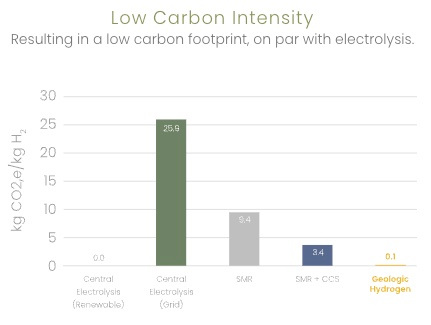
|
0.01 kg CO2/kg H2 vs 9.4 for SMR |
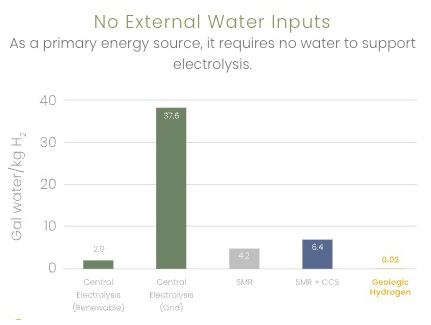 |
0.02 gal water/kg H2 vs 2.9 for electrolysis (renewable) |
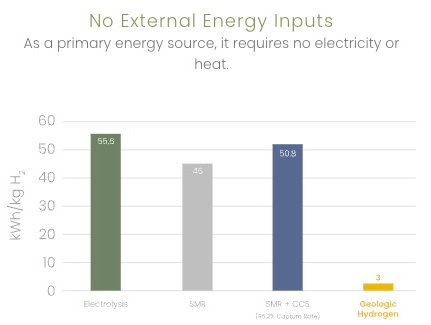 |
3 kWh/kg H2 vs 55.6 for electrolysis (renewable) |
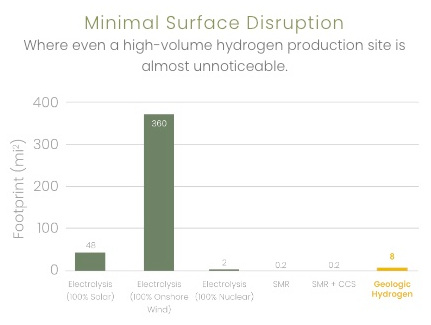 |
8 sq. mi vs 48 for electrolysis (solar) |
Natural Hydrogen Energy LLC
Natural Hydrogen Energy LLC is a Denver CO based company where Viacheslav Zgonnik is CEO. He is author of the 2020 Zgonnik Report below.
- "Hydrogen is generated by a natural geochemical process inside the Earth’s crust, and because of this it is a sustainable and inexhaustible source. Moreover, helium is often present in high concentrations within hydrogen accumulations."
- "Instead of drilling for fossil fuel we drill directly for natural hydrogen and associated helium".
Gold Hydrogen, Australia
Gold Hydrogen Limited is based in Brisbane, Australia.
- "Gold Hydrogen finds active natural hydrogen system in Australia" - South East Asia Iron and Steel Institute - November 2023
- "The Brisbane-based firm has now drilled 1,005 metres of so-called Ramsay 1 – Australia’s first natural hydrogen exploration well. Preliminary laboratory analysis has returned an air-corrected hydrogen concentration of 73.3% at a depth of 240 metres below ground level, in line with the 76% hydrogen historically reported in the well."
Natural Hydrogen in NM
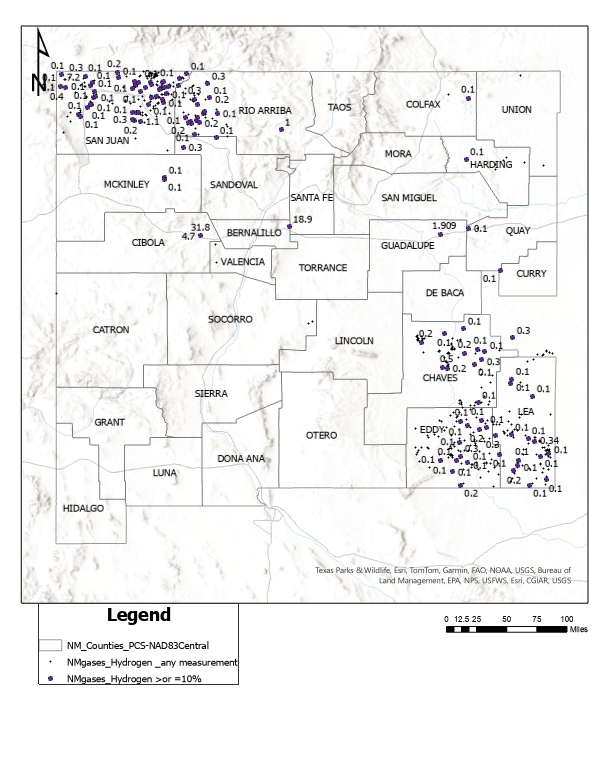
There is limited data available for the occurrence of geologic hydrogen in New Mexico. In a report on:
- Helium in New Mexico - Ronald Broadhead and Lewis Gillard - New Mexico Bureau of Geology & Mineral Resources - 2004
some data was collected, from oil and gas wells. Of the 937 samples analyzed, the highest concentrations found for hydrogen were in the San Andres, Madera and Hemorsa reservoirs with the following compositions:
| Reservoir | total hydrocarbons % | helium % | oxygen % | argon % | hydrogen % | nitrogen % | CO2% | Total % |
|---|---|---|---|---|---|---|---|---|
| San Andres | Tr | 2 | Tr | O,6 | 31.8 | 44.1 | 21.4 | 99.9 |
| Madera | 0.1 | 0.13 | 0 | Tr | 18.9 | 2.1 | 78.7 | 99.93 |
| Hermosa | 17.5 | 0 | 2.3 | 0.3 | 11.5 | 68.6 | 0 | 100.2 |
It must be noted that it's not known if all the 937 samples were analyzed for hydrogen, since some analytical methods rely on hydrogen as a carrier gas.
An additional source of information is this map of hydrogen, including analyses of over 10%, in New Mexico from the USGS:
No data yet is available on the fate of co-produced hydrogen.
In The News
Natural hydrogen in significant quantities has been identified in geological strata in Mali, Africa. Such hydrogen is sometimes known as either a 'White' or 'Gold' color. In the study of geology, it is unsurprisingly referred to as geologic hydrogen. Recent studies now indicate substantial quantities may be available in quite pure forms around the world.
- "Hidden Hydrogen: Does Earth hold vast stores of a renewable, carbon-free fuel?" - Science - February 2022.
- "There might be enough natural hydrogen to meet burgeoning global demand for thousands of years, according to a U.S. Geological Survey (USGS) model that was presented in October 2022 at a meeting of the Geological Society of America."
Another suggestion from the work is that hydrogen may be made continuously as water seeps or migrates into the underground rock formations, accumulating hydrogen under the geological and impermeable caps.
There is much work still to do to identify suitable locations for hydrogen prospecting, but the promise of another string in the bow of climate crisis solutions is encouraging as reported in The Guardian:
The report describes progress in finding what might be out there and where.
Recently, the New York Times reports potentially large reserves of white hydrogen in France:
- "It Could Be a Vast Source of Clean Energy, Buried Deep Underground" - NYT December 2023
- "In Lorraine, the scientists said their tests suggested that 46 million to 260 million metric tons of natural hydrogen could be lurking beneath the [abandoned] coal mines."
While continuing to push Natural Gas, even the American Gas Association is taking notice of Natural Hydrogen:
- "Natural Hydrogen Has Been Underestimated" - AGA - December 2023
For an updated overview that references the Zgonnik work:
- "Natural Hydrogen: A Potential Clean Energy Source Beneath Our Feet" - Yale Environment 360 - January 25, 2024
and
- "'There is enough natural hydrogen underground to meet all demand for hundreds of years', says US government agency" - Hydrogen Insight - February 19, 2024
- "Geologists signal start of hydrogen energy ‘gold rush’" - Financial Times - February 18, 2024
The Zgonnik Report
In 2020 the world wide occurrences of natural hydrogen of over 10% in purity were extensively documented in Zgonnik's report:
- "The occurrence and geoscience of natural hydrogen: A comprehensive review" - Earth Sciences Review - April 2020
Viacheslav Zgonnik, a Ukraine-born geochemist has conducted the most detailed review of the scattered scientific literature on hydrogen.
Zgonnik is pioneering hydrogen exploration in the U.S. with the Denver-based start-up Natural Hydrogen Energy where he is CEO.
The report collects together the findings and reports from a wide variety of researchers and authors under the headings:
- 1 Introduction - 1
- 2 Summary and classification of hydrogen discoveries - 3
- 2.1. Hydrogen as a free gas - 3
- 2.2. Diffusive flow - 9
- 2.3. Relationship of hydrogen with faults and with some noble gases - 12
- 2.4. Periodic variations in concentration - 14
- 2.5. Hydrogen in inclusions -16
- 2.6. Hydrogen dissolved in ground water - 20
- 3. Origin - 21
- 3.1. Deep-seated hydrogen - 21
- 3.2. Serpentinization - 28
- 3.3. Other reactions in minerals - 30
- 3.4. Water radiolysis - 31
- 3.5. Biological activity - 32
- 3.6. Atmospheric hydrogen - 33
- 3.7. Volcanoes and hydrothermal systems - 34
- 3.9. Anthropogenic sources - 35
- 3.8. Decomposition of organic matter - 35
- 4. Total global hydrogen budget and hydrogen cycle - 35
- 5. Hydrogen and natural phenomena - 37
- 5.1. Hydrogen as a component of Earth degassing - 37
- 5.2. Hydrogen and life - 38
- 5.3. Atmosphere - 39
- 5.4. Earthquakes - 41
- 5.5. Oil, gas and minerals - 42
- 6. Conclusions - 43
- References - 44 - 51
Some Key TakeAways
- As the author bemoans, there is insufficient effort to seriously prospect for more natural hydrogen reserves. While in 2018, there were 18 exploratory wells drilled in search of hydrogen, most discoveries of hydrogen are accidental and not intentional.
- Pure hydrogen occurs naturally per "documented evidence from wells flowing hydrogen with the rate of up to 100,000 m3 per day in East Siberia."
- Hydrogen's "discovery in significant quantities has shown that it is likely to be more concentrated at depth, with the largest accumulations being found in Precambrian basement, which is seldom targeted during drilling for conventional oil and gas."
- Because "hydrogen is colorless, odourless and non-toxic [it] may explain why hydrogen seepages have not been recognized earlier."
- "It is not accurate from a chemical point of view to consider only that part of a [geologic] reaction where hydrogen is generated and ignore the oxygen side of the equation."
- "The only well-examined process for the occurrence and genesis of hydrogen is serpentinization."
- "Numerous studies propose that the interior of the planet could be hydrogen-rich. The behaviour and habitat of hydrogen at depth remains almost an unknown subject and researchers should give specific attention to a deep-seated source for hydrogen."
- "Many studies have already demonstrated that the Earth’s core and mantle could contain significant quantities of hydrogen stored in the form of hydrides, most likely since the formation of the planet."
- "Moreover, deep-seated hydrogen could represent an inexhaustible source of energy."
- "It is important to note, that unlike natural gas, geologic hydrogen is a carbon-free, sustainable resource, which is constantly being generated underground. The natural processes producing hydrogen have been active since primordial times and will continue for millions of years in the future. Because of this natural hydrogen can be classified as a renewable source of energy.
For more discussion on the value of hydrogen as an energy source, rather than a vector (energy carrier) see:
- “Natural hydrogen, the next revolution? An inexhaustible and non-polluting energy, it does exist!”
- - Available from the International Atomic Energy Agency (IAEA)
- - Prinzhofer and Deville, 2015 - language: French,
- - Original title: Hydrogene naturel - La prochaine revolution energetique? 'Une energie inepuisable et non polluante, cela existe'
Geological Process and Origins
Geological processes may make such reserves virtually endless. Geologic hydrogen as been measured in:
- natural seeps
- oil & gas wells
- volcanic activity
The origins of geologic hydrogen have been hypothesised as:
- degassing of deep-seated hydrogen from the Earth’s core and mantle,
- the reaction of water with ultrabasic rocks or serpentinization,
- contact of water with reducing agents in the mantle,
- the interaction of water with freshly exposed rock surfaces,
- decomposition of hydroxyls in the lattice structure of minerals,
- natural radiolysis of water,
- decomposition of organic matter,
- biological activity,
- anthropogenic activities,
- the oxidation of ferrous (iron-II) minerals by water,
- the decomposition of hydrite minerals dating back to the earth's formation.
Some evidence of the latter comes from genetic analysis of primitive life forms that possess genes to live off the energy of hydrogen's oxidation.
Purple Hydrogen (Bio-Hydrogen, Biomass Hydrogen)
There are several ways hydrogen can be produced from biomass as suggested by the US Department of Energy "Hydrogen Production: Microbial Biomass Conversion"
Direct hydrogen fermentation uses microbes to break down organic molecules, and along with enzymes, makes hydrogen. The process is currently relatively slow with low yields, both are areas of continuing research.
Microbial Electrolysis Cells use the energy from microbial decomposition or organic matter along with a small electric current to produce hydrogen.
For continuing research see:
- "Bacteria Could Provide Cheap, Clean Hydrogen" - Technology Networks - October 2019
Since bio-hydrogen doesn't fit under the other 'colors' of hydrogen, while it can be classified as renewable, we have suggested it be 'purple' hydrogen. Generally, we shouldn't cut down forests to make wood for fuel since forestation, reforestation and afforestation are all natural carbon capture processes. Bio-mass hydrogen then has to be evaluated on the basis of how it contributes to reducing GHG emissions without it impacting the food chain.
Water Consumption of 'Green' vs 'Blue' Hydrogen
In the article:
- "Hydrogen production in 2050: how much water will 74EJ need?" - EnergyPost - July 2021
on research into how much water will be needed in the production of hydrogen through electrolysis (green hydrogen):
- It appears that total water use involved in producing hydrogen by electrolysis is about 32 kg H20/kg H2 and 22 kg H20/kg H2 when produced using electricity from photovoltaic cells or wind turbines, respectively (including electricity production, water purification and the electrolytic reaction itself).
- In steam methane reforming (methane reacting with water under heat and pressure), total water consumption (including water consumed in the reaction itself as well as water use during production of natural gas to heat the reaction, ranges from 7.6-37 kg H20/kg H2, with an average of about 22. The higher water consumption figure results, at least in part, when the natural gas used (for both methane for the reaction and to power the reaction) is derived from (fracked) shales.
- From a chemistry standpoint, SMR produces twice as much hydrogen per molecule of water consumed than does electrolysis, but it takes a substantial amount of water to produce the methane used in the process, so the net water use is not that different. But, electrolysis (theoretically) produces no CO2 whereas SMR does, both in the chemical reaction itself and in burning fossil fuels to generate heat to drive the reaction. Hence the need for extensive (and currently unachievable) levels of carbon sequestration to make 'gray' hydrogen 'blue'.
Some quotes from the article's summary:
"Assuming the world is using over 70EJ of electrolytic hydrogen by 2050, the water consumption will be about 25 bcm. That is relatively small compared with the global figure of 2,800 bcm for agriculture (the largest consumer), 800 bcm for industrial uses, and 470 bcm for municipal uses."
"the numbers suggests that water consumption shouldn’t be a major barrier for scaling up renewable hydrogen"
The report also notes that water has to be desalinated for electrolysis to avoid degradation of the electrolysis cells. And then, that water costs including treatment would only cost 2% of a best case total renewable cost of $2-3/kg H2. Fossil fuel hydrogen today (2021) is available at $1.80/kg H2, some current renewable numbers are up to $5/kg H2. Under the Biden Admin the DOE has a target to get costs down to $1/kg H2 by 2030.
Stoichiometrically, it takes 9 kg of water to make 1 kg hydrogen and 8 kg of oxygen through the 'decomposition' of water.
Oxygen: a Byproduct of Electrolysis (Green Hydrogen)
Worldwide Oxygen Production
When electrolyzing water for hydrogen it of course produces pure oxygen at the same time. Wikipedia reports the industrial production of oxygen via a number of processes was 100 Mtpa in 2003. The economic impact of renewable hydrogen and it's cogenerated oxygen very much needs to take this into account.
At $10/kg, the 2050 forecast demand for US' 41 Mtpa H2 comes with 41 * 16/2 Mtpa O2 = 328 Mtpa O2 which would on the face of it be worth $3,280 Billion/yr.
Cryogenic oxygen prices (see below) are reported around $0.10/kg O2, so depending on many variables (quantity, location, etc.) oxygen prices can vary considerably from 10c/kg to almost $2000/kg retail.
Utilizing Byproduct Oxygen
In the report:
the researchers at the Nagoya University, Japan, review the economic impact of byproduct oxygen on renewable hydrogen production.
An Italian study:
shows how medical centers could benefit from cogenerated oxygen from renewable hydrogen electrolyzers.
PlugPower a company focused on electrolyzer and fuel cell technologies reports it vents the oxygen from its electrolyzers to the air.
Retail Oxygen
Oxygen can be bought in canisters in stores under such brand names as 'Oxygen Plus' or 'Boost Oxygen' for 'recreational' purposes. As of Feb 2022 a 6 pack, each with 11 liters of oxygen at NTP costs about $90. At 1.429 gm/litre at NTP, the 66 liters cost the equivalent of $1,958/kg!
Cryogenic Oxygen
Traditional industrial oxygen is produced by a cryogenic process to separate oxygen and other gasses from air. Consultancy company Thunder Said Energy reported:
- Cryogenic air separation: costs and energy economics? - captured March 2024.
- "Energy consumption of air separation units is also estimated in the data-file, ranging from 150-800 kWh/ton of oxygen. The numbers can vary. Our estimates are built up using both bottom-up thermodynamic calculations, and a survey of data-points reported by companies and technical papers, tabulated in the data-file."
- "We estimate an oxygen price of $100/ton is sufficient for a new air separation unit to generate a 20% IRR. However, this includes help from selling nitrogen and argon."
150-800 kWh/ton oxygen at 2000 lb/ton corresponds to $0.16-0.88 kWh/kg oxygen. $100/ton selling prices equates to $0.11/kg oxygen.
As of March 2024, The full report is available for $499.
High Volume Oxygen suggests a price of $0.07-0.10/kg oxygen.
Hydrogen - A Greenhouse Gas too?
Studies are now indicating hydrogen may have a stronger greenhouse gas impact than previously thought according to studies in the UK.
- "Hydrogen 'twice as powerful a greenhouse gas as thought before': UK government study" - Recharge - April 2022
The 75-page report, Atmospheric Implications of Increased Hydrogen Use, explains that H2 is an indirect greenhouse gas, since it reacts with other greenhouse gases in the atmosphere to increase their global warming potential. The researchers calculate reactions that also occur in the second lowest layer, the stratosphere, concluding: “we estimate the hydrogen GWP(100) [that is, over a 100-year period] to be 11 ± 5; a value more than 100% larger than previously published calculations.” A previous study from 2001, which has been frequently cited ever since, put the GWP of hydrogen at 5.8. The report also discussed possible leakage rates and recommends they be kept to a minimum.
Hydrogen in and of itself is not considered a greenhouse gas so if there were no other GHGs in the atmosphere there would be no effect.
Referenced reports are:
- Atmospheric implications of increased Hydrogen use - Nicola Warwick, Paul Griffiths, James Keeble, Alexander Archibald, John Pyle, University of Cambridge and NCAS and Keith Shine, University of Reading - April 2022
- Fugitive Hydrogen Emissions in a Future Hydrogen Economy - Frazer-Nash Consultancy - March 2022
Town Gas
Town gas, sometimes referred to as Coal Gas, can be made from naphtha instead of coal. Hong Kong town gas is made from naphtha and natural gas. According to their website it has the following composition:
| Component | Percent |
|---|---|
| Carbon Dioxide | 16.3% – 19.9% |
| Carbon Monoxide | 1.0% – 3.1% |
| Methane | 28.2% – 30.7% |
| Hydrogen | 46.3% – 51.8% |
| Nitrogen and Oxygen | 0% – 3.3% |
Historically, town gas was made from coal. It's toxic nature (carbon monoxide levels) led to the suicide use of ovens. After converting to natural gas the UK saw an overall drop in suicides of a third.
Town gas can sometimes be composed of hydrogen, carbon monoxide, methane, ethylene and volatile hydrocarbons together with small quantities of non-calorific gases such as carbon dioxide and nitrogen. It is made from coal and steam and was first introduced in the UK in 1790 for lighting.
The UK converted from town gas to natural gas over a period from 1967 to 1977 that included installing different-sized burner jets to give the correct gas/air mixture. Mostly the conversion was uneventful.
The composition of UK town gas was typically:
| Component | Percent |
|---|---|
| Hydrogen | 50% |
| Methane | 35% |
| Carbon Monoxide | 10% |
| Ethylene | 5% |
Ref. Wikipedia Town Gas
Natural Gas
Chemical Composition of Natural Gas
Locally, natural gas is a naturally occurring gas mixture, consisting mainly of methane sourced from supply basins in the Four Corners and Permian Basin in New Mexico.
The following composition is an overall system average and may vary from the typical values listed depending on location.
| Component | Typical Analysis (mole %) | Range (mole %) |
|---|---|---|
| Methane | 94.7 | 87.0 - 98.0 |
| Ethane | 4.2 | 1.5 - 9.0 |
| Propane | 0.2 | 0.1 - 1.5 |
| iso - Butane | 0.02 | trace - 0.3 |
| normal - Butane | 0.02 | trace - 0.3 |
| Propane | 0.2 | 0.1 - 1.5 |
| iso - Pentane | 0.01 | trace - 0.04 |
| normal - Pentane | 0.01 | trace - 0.04 |
| Hexanes plus | 0.01 | trace - 0.06 |
| Nitrogen | 0.5 | 0.2 - 5.5 |
| Carbon Dioxide | 0.3 | 0.05 - 1.0 |
| Oxygen | 0.01 | trace - 0.1 |
| Hydrogen | 0.02 | trace - 0.05 |
Helium in Natural Gas
A number of natural gas fields have also accumulated Helium gas. Such finds make it a bonus to the well owners as helium can bring a premium price in the open market. Some natural gas fields can have as much as 7% or more. Helium can occur in reservoirs without natural gas. Major helium reservoirs have been found in Tanzania in Africa:
- Massive Underground Helium Reserve Found in Tanzania - Live Science - October 2017
Properties of Natural Gas
A selection of physical properties of natural gas are included below.
| Property | Typical Analysis | Range |
|---|---|---|
| Specific Gravity | 0.58 | 0.57 - 0.62 |
| Gross Heating Value (MJ/m3), dry basis * | 38.8 | 36.0 - 40.2 |
| Wobbe Number (MJ/m3) | 50.9 | 47.5 - 51.5 |
﹡ The gross heating value is the total heat obtained by complete combustion at constant pressure of a unit volume of gas in air, including the heat released by condensing the water vapour in the combustion products (gas, air, and combustion products taken at standard temperature and pressure).
Sulphur may vary from 3 - 6 mg/m3 depending on the field.
Water vapour may be less than 65 mg/m3 and can vary from 16 - 32 mg/m3 depending on the field,
Typical combustion properties of natural gas:
Ignition Point: 564 oC * Flammability Limits: 4% - 15% (volume % in air) * Theoretical Flame Temperature (stoichiometric air/fuel ratio): 1953 oC * Maximum Flame Velocity: 0.36 m/s *
﹡ The properties shown may vary depending on the location. Information provided is from the Ortech Report No. 26392, Combustion Property Calculations for a typical Union Gas Composition, 2017.
Industrial Hydrogen Uses
The WHA(World Hydrogen Association?) reports on industrial hydrogen uses worldwide: 25% petroleum refining, 55% ammonia (thence to fertilizer), 10% methanol and 10% other that includes fuel cells, glass, electronics, medical, food and metallurgical uses. In a transition away from fossil fuels the petroleum refining segment will decline but still leave considerable demand in other sectors.
Hydrogen As a Fuel
Transportation
Many applications of hydrogen in Hydrogen Powered Transportation are being pursued. Hydrogen can be used in fuel-cells and in combustion engines. In terms of sales volume quantities are minimal compared to making ammonia.
NOx Emissions from Burning Hydrogen in Air
The amount of Nitrogen Oxides NOx emissions when burning hydrogen in air either for industrial or residential purposes depends on many factors. NOx are greenhouse gases so their generation should be minimized. NO2 has a molecular weight of 46 making it denser than air (avg MW 29.87) while NO has a molecular weight of 30, about the same as air, N20 has a molecular weight of 44.
Some proposals include adding hydrogen to fuel gas in for example industrial furnaces. NOx generation is discussed in this article from The Chemical Engineer and discusses the factors involved, e.g. burner design, flame temperature and such. Since NOx emissions are legally constrained in some jurisdictions, burner design plays an important role to meet such specification.
Diesel and gasoline internal combustion engines are a major source of NOx emissions where it is mitigated by catalytic converters.
A three-way catalyst can cut CO, HC and NOx by over 99% if the air to fuel ratio is accurately controlled according manufacturer Johnson Matthey.
Hydrogen Storage
Pressurized Tanks
The hydrogen storage tanks used for high-pressure gaseous hydrogen storage can be roughly divided into five types:
- Type I: metallic pressure vessel,
- Type II: metallic liner hoop wrapped with CFRP
- Type III: metallic liner fully wrapped with CFRP
- Type IV: polymer liner fully wrapped with CFRP
- Type V: has 20% less weight than type IV, is made of composites without a liner
Vehicle tanks are designed to operate at 10,000 psi but rated for 25,000 psi.
CFRP - carbon fiber-reinforced polymer
Permeation Rates
Permeation rates of hydrogen in various storage configurations has been reported. During some safety investigations, researchers reported the following leakage rates:
"Based on the data provided by the manufacturers, the hydrogen permeation rates of the hydrogen storage vessels of vehicle A and B were approximately 2.35 mL/min and 1.80 mL/min, respectively."
One presumes the volumetric measurements corresponded to standard temperature and pressure conditions (STP).
Hydrogen tanks in trains, boats, planes and cars are made of carbon fiber and run around 10,000 psi their NWP (normal working pressure) but designed for 25,000 psi. Apparently there are at least Type III and Type IV hydrogen storage tanks in use.
Meanwhile, at NWP and ambient temperature, ISO 19881 requires that the steady-state permeability of Type IV hydrogen storage tanks in the system should be less than 6 Ncm3/h/L. (see "Review of the Hydrogen Permeability of the Liner Material of Type IV On-Board Hydrogen Storage Tank".
The density of hydrogen at NTP (68 deg F 1 atm) is 0.08376 kg/m3. A typical(?) hydrogen vehicle gets 70 miles/kg H2, so a 300 mile range requires a tank capacity of 4.28 kg. A 10,000 psi hydrogen tank will be about 200 liters or 3-4 times the volume of gasoline tanks typically found in internal combustion engine cars.
So for an automobile tank to meet the ISO standard it will lose hydrogen, when fully pressurized, no faster than the rate of:
6 Ncm3/h/L * 200 L * 30 (days/mon) * 24 (hr/day) = 864,000 cm3/month
At a density of 0.08376 kg/m3 and with 1,000,000 cm3/m3, hydrogen losses are:
864,000 * 0.08376 / 1,000,000 kg/mo = 0.0724 kg/mo equivalent to 1.3% of a 5.6 kg charge per month.
The 2mL/min rate of the A and B vehicles above and a 200L tank, equates to about 2 mL/min * 60 /200 = 0.6 Ncm3/h/L or 10x lower than the standard demands, making their tanks lose only 0.13% of a charge per month.
The Toyota Mirai 2022 has a Type IV tank capacity of 5.6 kg H2 spread over three tanks running at a pressure of 70MPa (10,150 psi).
Metal Hydride Tanks
Metal hydride storage systems for hydrogen operate at much lower pressures than pure hydrogen tanks. For a discussion of the technology see:
- "How Powder Metal Hydrides Solve Safety And Size Challenges For Hydrogen Storage" - GKN Sinter Metals Engineering GmbH - Jan 2020
Some quotes:
"As an alternative to high-pressure storage systems, metal hydrides are a safe and controllable technology to store hydrogen at lower pressures in small spaces. This low-pressure concept works because the hydrogen molecules are chemically bonded within the metal compound structure and remain stable and nonhazardous at atmospheric pressure. Metal hydride storage systems typically operate at 10-40 bars, which is twenty times less than typical high-pressure systems. Once the hydrogen is needed, the desorption process begins by feeding thermal heat (45 – 65°C) so gas begins to flow outward. At this stage, pressures are down to around one to two bars."
Metal Hydride tanks are too heavy though for transportation applications.
Ammonia as Energy Carrier
While hydrogen's role in the process to make anhydrous ammonia is mostly part of the supply chain to chemicals and fertilizers. Ammonia,as an energy carriere, has a higher energy density, at 12.7 MJ/L, than even liquid hydrogen, at 8.5 MJ/L. Liquid hydrogen has to be stored at cryogenic conditions of –253 °C, whereas ammonia can be stored at a much less refrigeration energy-intensive –33 °C. And ammonia, though hazardous to handle, has a much narrower flammable range than hydrogen when mixed with air.
When used as a fuel the combustion produces only water and nitrogen. Ammonia has a GWP zero (0) and can also be used as a refrigerant replacing the many HFCs with GWP in the thousands. It is toxic, but doesn't have to be deodorized like natural gas, so well known safety precautions have to be practiced.
Anhydrous ammonia is distributed by pipeline and tankers for many industrial purposes. Note ammonia cleaners are a solution of ammonia hydroxide.
Hydrogen Pipelines
As an alternative to on site compression of gaseous hydrogen at hydrogen gas stations for fuel-cell vehicles fill-ups, an analysis of the economics of a high pressure hydrogen distribution pipeline shows cost savings over hydrogen delivery trucks. The report "Economic analysis of a high-pressure urban pipeline concept (HyLine) for delivering hydrogen to retail fueling stations" was developed by staff at the National Renewable Energy Laboratory (NREL) and independent contractors. The Green Car Congress site carries the same story.
The Texas Gulf coast has 1,600 km (1,000 miles) of hydrogen pipeline installed connecting 48 hydrogen plants according to ICIS. Operating pressures are not yet known.
In December 2023, the New York TImes reports European plans to build a network of hydrogen pipelines to distribute manufactured hydrogen to factories and fuel sites. The mosaHYc project is a starting point with 70 km of pipeline that was commissioned in 2022. It will be tied into the European hydrogen producer Lhyfe's new renewable hydrogen plant in Perl, west Germany, that the firm announced 11 July 2023. Lhyfe is expected to build a 70MW renewable hydrogen production plant.
Hydrogen Embrittlement of Pipelines
The mechanism behind hydrogen embrittlement can be complicated. Generally for hydrogen embrittlement to occur, a combination of three conditions are required:
- the presence and diffusion of hydrogen atoms or ions (i.e. not just the molecules)
- a susceptible material
- mechanical stress
Poor quality welding may contribute to the presence of 'susceptible materials'.
Steel with an ultimate tensile strength of less than 1000 MPa (~145,000 psi) or hardness of less than 32 HRC is not generally considered susceptible to hydrogen embrittlement. Very few steels have a tensile strength over 1000 MPa - One, 'Tool Steel-Cold Hardened', is up to 1200 MPa.
Hydrogen embrittlement in pipeline steel is not a problem in natural gas pipelines. 50% hydrogen in pipelines used to be the norm in the UK as Town Gas, probably for decades, before natural gas came on board and used the same distribution network.
Hydrogen Safety
An in depth review of the safety of hydrogen was presented at the 2nd Joint Summer School on Hydrogen and Fuel Cell Technologies, on 19 September 2012, Crete:
- Hazards related to hydrogen properties and comparison with other fuels - Ulster University - Vladimir Molkov, 2012
a briefer discussion of hydrogen safety is shown at:
- Dispelling Common Hydrogen Safety Myths - July 12, 2018
Hydrogen Markets
World Energy Transition Forecasts for Hydrogen
Forecasts by world consultants DNV in their Energy Transition Outlook Report 2023, predict that by 2050 over half of world hydrogen production will have converted to electrolysis, consuming 7.2% of their forecast 2050 world electrical power generation. For some context, about 95% of hydrogen is produced from fossil-fuels today and aluminum production is reported to consume about 5% of world electrical energy generation.
Worldwide Hydrogen Production
Meanwhile, (from a Nov 2012 article) 2019 total world production of pure hydrogen is 75 million metric tonnes. The same article reports:
"Electrolysis of water at ambient temperatures requires 50-55 kWh per kilogram of hydrogen produced* (hence 60% and potentially 70% efficient with improved catalysts)."
The power to make the world's 75 million tonnes of pure hydrogen via electrolysis is therefore 75,000,000,000 kg * 55 kWh/kg = 4,125,000 GWh.
From Wikipedia,
"As of the end of 2020, the United States had 97,275 megawatts (MW) of installed photovoltaic and concentrated solar power capacity combined."
If all the US installed solar power operated for 2000 hr/yr, it would generate 2000 * 97,275 MWh = 194,550 GWh. That in turn could then make 4.7% of the world's supply of hydrogen, insufficient for US purposes. At $1.8/kg H2, the corresponding annual gross income would be $75,000,000,000 * 0.047 *1.8 = $6.345 billion/yr.
US Hydrogen Demand
For a perspective on the US hydrogen demand see:
- Hydrogen’s Present And Future In The Us Energy Sector - Shearman & Sterling - October 2021
The report notes that 90% of the 10 Mtpa of US hydrogen production is from natural gas. To put it in perspective, current US solar power could produce about 3.525 Mtpa H2 (with no solar power going into the grid). If NM could grow production to meet that demand, the state could enjoy a gross income of $6.345 billion/yr. For comparison 2016 NM state revenues were $5.462 billion/yr (Ballotpedia). But there are lots of buts: we should back out hydrogen from chlorine production from the calcs, for example.
Meanwhile US demand for hydrogen is forecast to quadruple to 41 Mtpa by 2050.
US Hydrogen Electricity Demand
If all the US' demand for hydrogen was satisfied by green hydrogen production, according to Tom Solomon's (350NM) projections, for 100% energy production from renewables the US will need an installed base of 4,545 GW (by 2036). Operating at 2000 hr/yr that's 9,090,000 GWh, To meet the 2050 US demand for hydrogen of 41 Mtpa at 55 kWh/kg H2 then would require:
41,000,000,000 kg * 55 kWh/kg / 1,000,000 kWh/GWh = 2,255,000 GWh.
(Note, by 2050 improved technologies may exceed the 55 kWh/kg H2. 2000 hr/yr is 5.5hr/day 365 days a year. See Unbound Solar)
Based on a 2000 hr/yr operation, this would require increasing the installed renewable energy base by 1,127.5 GW an increase of 24.8% over Solomon's 2036 number. At $1.8/kg (current fossil hydrogen prices), 41 Mtpa H2 is worth $73.8 Billion/yr.
Corresponding Reductions in US GHG emissions
At 7 kg CO2/kg H2 on average for steam methane reforming (SMR), converting a US 2050 demand of 41 Mtpa to renewables saves 41 * 7 = 287 million tonnes/yr of CO2 emissions and an unknown amount of leaked potent methane emissions.
Annual Water Consumptions in NM
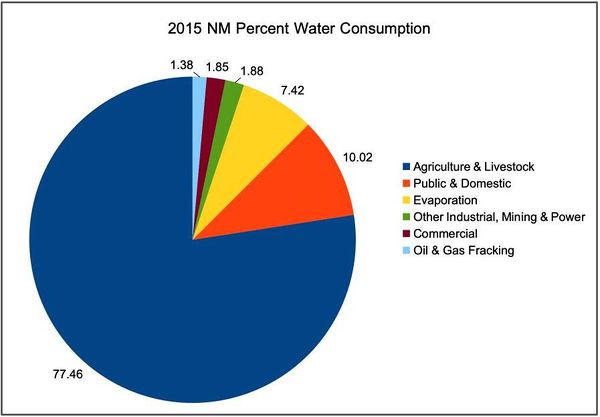
2015 NM Water Usage Categories
The following numbers are based on:
- * one acre-foot (AF) of water is 326,000 gals
- * 0.419 metric tonnes of hydrogen are made from 1 billion gals of water via electrolysis
For 2015, the State Engineer tabulated the following totals for the whole state of New Mexico:
- Total Withdrawals: 3,114,255 AF = 1,015 billion gals/yr - 100%
- Public & Domestic: 312,106 AF = 101.7 billion gals/yr - 10.02%
- Agriculture & Livestock: 2,412,111 AF = 786.3 billion gals/yr - 77.46%
- Commercial: 57,526 AF = 18.7 billion gals/yr - 1.85%
- Industrial, Mining & Power: 101,431 AF = 33.0 billion gals/yr - 3.26%
- Evaporation from reservoirs: 231,081 AF = 75.3 billion gals/yr - 7.42%
To put this in context the 2019 USGS FracFocus database contains an estimated fracking water usage of:
- NM Oil & Gas (fracking): 42.900 AF = 14 billion gals/yr - 1.38%
This corresponds to 1.38% of the state's 2015 total withdrawals and as noted above is enough to make 5.88 Mtpa H2 or over half US hydrogen market and corresponds to a 75 MW rated power unit at 50% OST. Compare this to the Xcel Energy's Sagamore Wind Project in Roosevelt County NM, which is rated at 522 MW.
What Impact Will Cannabis Growers Have on Water Usage?
Numbers are difficult to come by. An AP report "Questions raised about cannabis growers’ water use" from Feb 2019 suggests a cannabis farm typically consumes 3,000 gals/day. The Public Policy Institute of California in a June 2021 blogpost "How Does Cannabis Cultivation Affect California’s Water?" reported that there were 8,000 legal cannabis farms in California.
At that scale, which doesn't include illegal farms, the total water usage amounts to 8.76 billion gals/yr which is equivalent to 0.86% of 2015 NM total withdrawals.
While there currently are an unknown number of growers in NM, their water usage hasn't gone unnoticed per the January 2020 Albuquerque Journal report: State’s water takes a hit from cannabis farms.
How Much Water Will Regenerative Agricultural Practices Save?
Future water consumption numbers, or even those since 2015 may be impacted by the adoption of Regenerative Agriculture practices across the state. Numbers have so far been impossible to find but expectations are that agricultural water usages weil be much reduced from standard practices!
2015 NM State Engineer Water Usage Numbers
The 2015 data on the use of water across the state was compiled by the State Engineer:
- New Mexico Water Use By Categories 2015 - New Mexico Office of the State Engineer - May 2019
Report Summary
The population of New Mexico increased from 2,059,179 in 2010 to 2,099,856 in 2015, an increase of 40,677 or almost 2%.
In 2015, withdrawals for all water use categories combined totaled 3,114,255 acre-feet (AF) down 14% from 2005. Surface water accounted for 1,629,968 AF (52.34%) of the total withdrawals, and groundwater accounted for 1,484,287 AF (47.66%) of the total withdrawals. A summary of withdrawals for 2015 by category and source is provided below.
Public Water Supply accounted for 284,157 AF (9.12%) of the total withdrawals, consisting of:
- 87,399 AF (30.76%) of surface water
- 196,758 AF (69.24%) of groundwater
Self-Supplied Domestic accounted for 27,949 AF (0.90%) of the total withdrawals, consisting entirely of groundwater.
Irrigated Agriculture accounted for 2,376,065 AF (76.30%) of the total withdrawals, consisting of:
- 1,255,440 AF (52.84%) of surface water.
- 1,120,625 AF (47.16%) of groundwater.
Surface water diverted for irrigation resulted in off-farm conveyance losses in canals and laterals, which amounted to 425,618 AF (33.90% of the diversion total). � New Mexico Water Use by Categories 2015 ii New Mexico Office of the State Engineer Technical Report 55 Water Use and Conservation Bureau The total estimated acreage irrigated (TAI) on farms in 2015 was 749,769 acres. Approximately 226,870 acres (30.26%) were irrigated with surface water, 408,628 acres (54.50%) were irrigated with groundwater, and 114,271 acres (15.24%) were irrigated with a combination of groundwater and surface water. Total drip irrigation (TDA) accounted for 23,466 acres (3.13%), total flood irrigation (TFA) accounted for 340,780 acres (45.45%), and total sprinkler irrigation (TSA) accounted for 385,523 acres (51.42%). In some areas of the state, surface water was not sufficient to meet the irrigation demand.
Livestock accounted for 36,046 AF (1.16%) of the total withdrawals, consisting of:
- 2,904 AF (8.06%) of surface water.
- 33,142 AF (91.94%) of groundwater.
Commercial uses accounted for 57,526 AF (1.85%) of the total withdrawals, consisting of:
- 12,326 AF (21.43%) of surface water.
- 45,199 AF (78.57%) of groundwater.
Industrial uses accounted for 8,718 AF (0.28%) of the total withdrawals, consisting of:
- 0 AF (0%) of surface water.
- 8,718 AF (100%) of groundwater.
Mining accounted for 42,294 AF (1.36%) of the total withdrawals, consisting of:
- 1,141 AF (2.70%) of surface water.
- 41,153 AF (97.30%) of groundwater.
Power accounted for 50,419 AF (1.62%) of the total withdrawals, consisting of:
- 39,677 AF (78.69%) of surface water.
- 10,742 AF (21.31%) of groundwater.
Evaporation from reservoirs with a storage capacity of 5,000 AF or more amounted to 231,081 AF (7.42%) of total withdrawals.
2005 NM State Engineer Water Usage Numbers
The 2005 data on the use of water across the state was compiled by the State Engineer:
- New Mexico Water Uses by Category 2005 - New Mexico Office of the State Engineer - June 2008
Report Summary
In 2005, withdrawals for all categories combined totaled 3,950,398 acre-feet. Surface water accounted for 2,112,138 acre-feet (53.47%) of the total withdrawals; groundwater accounted for 1,838,260 acre-feet (46.53%) of the total withdrawals.
Public Water Supply accounted for 320,126 acre-feet (8.10%) of the total withdrawals. Surface water accounted for 42,092 acre-feet (13.15%) of the public water supply withdrawal.
Groundwater accounted for 278,034 acre-feet (86.85%). Self-Supplied Domestic accounted for 35,796 acre-feet (0.91%) of the total withdrawals. In this category, 100% of the withdrawals for domestic purposes were from groundwater sources.
Irrigated Agriculture accounted for 3,075,514 acre-feet (77.86%) of the total withdrawals. Surface water accounted for 1,730,927 acre-feet (56.30%) of irrigation withdrawals. Groundwater withdrawals totaled 1,344,587 acre-feet (43.70%).
Surface water diverted for irrigation resulted in off-farm conveyance losses in canals and laterals, which amounted to 608,901 acre-feet (35.12%).
The total acreage irrigated (TAI) on farms in 2005 was 875,415. Approximately 279,665 acres (31.95%) were irrigated with surface water; 464,177 acres (53.02%) were irrigated with groundwater; and 131,573 acres (15.03%) were irrigated with a combination of ground and surface water. Drip irrigation (TDA) accounted for 18,875 acres (2.16%); flood (TFA) for 448,599 acres (51.24%); and sprinkler (TSA) for 407,941 acres (46.60%).
In some areas of the state, surface water was not sufficient to meet the irrigation demand. Livestock accounted for 57,009 acre-feet (1.44%) of total withdrawals. Surface water accounted for 3,279 acre-feet (5.75%) of withdrawals and groundwater for 53,730 acre-feet (94.25%).
Commercial uses accounted for 40,578 acre-feet (1.03%) of total withdrawals. Surface water accounted for 1,496 acre-feet (3.69%) of the withdrawals, and groundwater for 39,082 acre-feet (96.31%).
Industrial uses accounted for 18,251 acre-feet (0.46%) of total withdrawals. Surface water accounted for 1,967 acre-feet (10.78%) of the withdrawals and groundwater for 16,284 acre-feet (89.22%).
Mining accounted for 60,189 acre-feet (1.52%) of total withdrawals. Surface water accounted for 1,438 acre-feet (2.40%) of the withdrawals and groundwater for 58,751 acre-feet (97.61%).
Power accounted for 63,642 acre-feet (1.61%) of total withdrawals. Surface water accounted for 51,646 acre-feet (81.15%) of withdrawals and groundwater for 11,996 acre-feet (18.85%).
Evaporation from reservoirs with a storage capacity of 5,000 acre-feet or more amounted to 279,293 acre-feet (7.07%) of total withdrawals.
Problems with Oil & Gas Field Waters
Management of these waters is challenging not only for industry and regulators, but also for landowners and the public because of differences in the quality and quantity of produced and flowback water, varying infrastructure needs, costs, and environmental considerations associated with oil and gas fields' water disposal, storage, and transport.
Hydrogen Hubs Background
What is a Hydrogen Hub?
According to the NM Environment Department Clean Hydrogen Fact Sheet, a
- "Clean Hydrogen Hub is a network of clean hydrogen producers, and connective infrastructure. It contains all facets of a hydrogen ecosystem: production, delivery, storage, and end uses. A clean hydrogen hub must be self-sustaining after DOE's initial investment."
A definition of 'clean hydrogen' is not specified, but 'steam methane reformation with carbon capture is included as one wary to make it. The fact sheet sheds no light on the technical developments necessary to make either selectrolysis or carbon capture cost effective with today's Steam Methane Reforming process.
The Department of Energy has similar and more details in their description of Regional Clean Hydrogen Hubs. Namely
- Clean hydrogen hubs will create networks of hydrogen producers, consumers, and local connective infrastructure to accelerate the use of hydrogen as a clean energy carrier that can deliver or store tremendous amounts of energy.
- The production, processing, delivery, storage, and end-use of clean hydrogen, including innovative uses in the industrial sector, are crucial to DOE’s strategy for achieving President Biden’s goal of a 100 percent clean electrical grid by 2035 and net-zero carbon emissions by 2050.
Again, a definition of 'clean hydrogen' is not specified.
Federal Incentives
From White House Launches ‘Generational’ $7 Billion Hydrogen Plan - Bloomberg - Sept 22, 2022
- The Energy Department has opened up applications for $7 billion to establish up to 10 regional hydrogen hubs, part of a broader road map unveiled Thursday that officials described as essential to lowering emissions in industrial sectors such as energy, transportation, steel, and cement.
- The hub program—established by Congress in the infrastructure bill passed last November—was announced during the Energy Department’s Clean Energy Ministerial and Global Clean Energy Action Forum in Pittsburgh, where government officials and industry executives discussed hydrogen’s role in meeting climate goals.
- The department plans to select at least four regional hubs, with at least one from “green” hydrogen produced by renewable energy; one from “blue” hydrogen sourced from natural gas and using carbon capture and storage; and one “pink” hydrogen project from nuclear power.
- The department is seeking geographic diversity and expects to see applications that include broad groups of hydrogen stakeholders: companies, government agencies, producers and consumers, pipelines, and transportation companies, said Kelly Cummins, acting director and principal deputy director for the Office of Clean Energy Demonstrations, which is managing the program.
- No major changes were made to the department’s hydrogen hub strategy compared with a notice of intent released in June, Cummins said. Concept papers are due by Nov. 7, 2022, and full applications are due by April 7, 2023.
Western Inter-States Hydrogen Hub (WISHH)
New Mexico with neighboring states in February 2022 entered a memorandum of understanding (MOU) to develop a proposal to the Department of Energy for a fossil-fuel based hydrogen hub. It's not without controversy being based on "blue" hydrogen as noted in the MOU commentary from the Western Environmental Law Center. The four states in the 'consortium' are Colorado, New Mexico, Utah, and Wyoming.
In November 2022 WIH2 Inc. - a totally owned subsidiary of Atkins, which in turn is a member of the SNC-Lavelin Group, published their Concept Paper identifying major partners and Atkins as the major contractor.
In 2023 the DoE announced the WISSH project a joint collaboration between Wyoming, Utah, New Mexico and Colorado, would not be selected for funding as a Hydrogen Hub:
- "Colorado loses bid to build a federally funded regional hydrogen hub" - CPR News - October 2023
Renewable Hydrogen Opportunities for NM (Ed)
If New Mexico were to overbuild solar and wind capacity it's possible to estimate how much excess power could be generated, converted to hydrogen and sold to the industrial users. The industrial gasses sector is orders of magnitude bigger than anticipated energy storage and transportation needs. Such a proposition needs to be compared with just selling the power in terms of costs, locations and jobs. Which is quicker and cheaper a) export H2 in trucks or pipelines or b) build transmission lines to export the energy?
Hydrogen Industry Conferences
The World Hydrogen 2023 Summit & Exhibition, 9-11 May 2023, the leading global platform dedicated to hydrogen industry advancement is produced by the Sustainable Energy Council (SEC) in partnership with the Province of Zuid-Holland, the City of Rotterdam and the Port of Rotterdam, World Hydrogen 2023 is the place to meet with government and private sector leaders to showcase, discuss, collaborate and do business, driving the hydrogen industry forward.
Hydrogen Tech World Conference & Exhibition Essen, 4–5 April 2023 - The Hydrogen Tech World Conference will focus primarily on the technologies used in green hydrogen production, storage, and transportation.
Special Interest Groups on Hydrogen
The UK Institution of Chemical Engineers, magazine The Chemical Engineer hosts a hydrogen special interest group that examines a wide variety of issues associated with a "hydrogen economy".
Summary
Renewable hydrogen (made by electrolyzing water with renewable energy, aka 'green' hydrogen))
- is not a problem as far as water usage is concerned,
- saves water when combined with curtailing fracking as methane demands are replaced,
- does not have a leakage (permeation) problem in transportation applications,
- is big business when decarbonizing industrial hydrogen (SMR) production,
- makes big cuts in GHG emissions; CO2 from SMR and methane emissions,
- brings additional economic benefits from the utilization of by-product oxygen.
- is possible by repurposing the states fracking water demands to green hydrogen and would meet over half the US demand for hydrogen.
While, the power requirements to meet US industrial hydrogen demand may be only as much a 1/4 of total needs, building out a renewable hydrogen business in NM could go along way to meeting our state budgetary needs, depending on taxation and public ownership issues. Continuing technological improvements in efficiencies and performance in the electrolyzer, fuel-cell and storage fields make this picture improve all around. Monetizing the coproduced oxygen may make process very profitable.
Geologic hydrogen, found at high purities in the right geological formations. can be accessed with established drilling technology. Geologic hydrogen can provide a low water, low energy demand, non-polluting, possibly inexhaustible supply.
Physical Properties
Density of oxygen (STP) = 1.429 g/l
Density of hydrogen (STP) = 0.08988 g/L
1 g/L = 1 kg/m3
1 bar = 0.98692327 atmosphere
Abbreviations
bcm = billion cubic metres
CFRP = Carbon fiber reinforced plastic or polymer
EJ = exajoules (1 EJ is about 947.8 trillion BTUs, or 278 thousand GWh)
ICE = Internal Combustion Engines
MPa = megaPascals (1 MPa = 145.04 psi)
NTP = Normal Temperature and Pressure (68 deg 1, 1 atm)
psi = pounds per square inch
STP = Standard Temperature and Pressure (0 deg C, 1 bar)
Ncm3/h/L = Normal cubic centimeters per hour per liter (of storage capacity)